Introduction
Metal-organic frameworks (MOFs) present a class of materials showing properties akin to both solids and molecular systems. They consist of inorganic metal or metal-oxide clusters (nodes) connected through organic molecules (linkers), giving rise to porous, highly tunable frameworks. Their porous nature, with internal surface areas of >1000 m2g-1, and chemical tunability, through the choice of nodes and linkers, makes them versatile materials that are receiving a rapidly growing interest with a large focus on industrial, chemically oriented processes, such as catalysis, gas separation and gas storage.
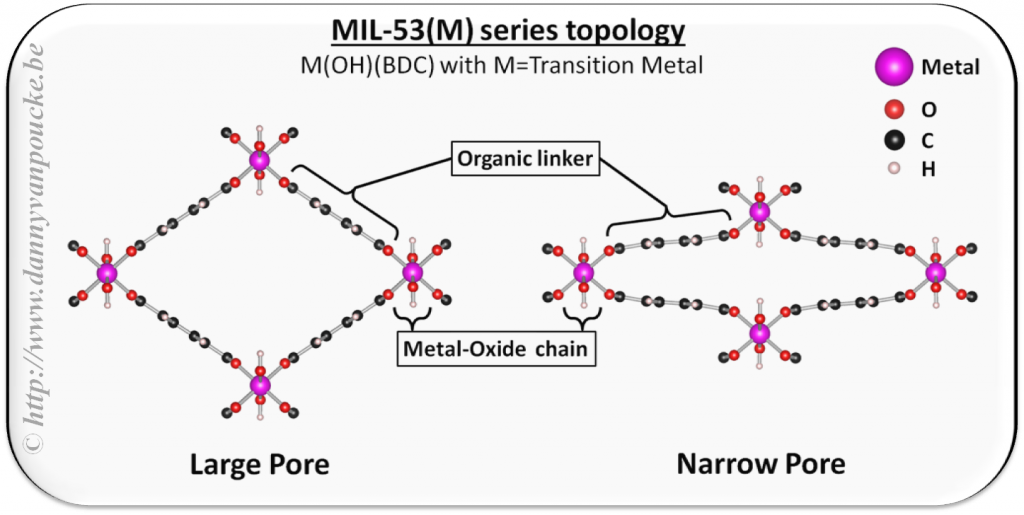
Figure 1: Ball-and-stick representation of the MIL-47/53 class of breathing MOFs. These MOFs are known to present a structural phase transition from a large-pore (left) to a narrow-pore (right) geometry under the influence of external stimuli such as pressure, temperature, or gas-sorption.
In this project, I focus on MOFs with the MIL-47/MIL-53 topology (cf. Figure 1), which contain 1D transition-metal oxide chains. These MOFs belong to the class of so-called breathing MOFs because they can reversibly switch between a large-pore (LP) and narrow-pore (NP) configuration under the influence of guest molecules, temperature or pressure. The presence of transition metals on the other hand make these MOFs of interest for magnetic and/or multiferroic applications. I am studying the mechanical and electronic properties of such MOFs using ab initio methods, and found these MOFs to present an interesting test bed for quasi-1D physics.
Related Publications
-
J. Phys. Chem. C 117(44), 22784-22796 (2013),
doi: 10.1021/jp406835n -
Beilstein J. Nanotechnol. 5, 1738-1748 (2014),
doi: 10.3762/bjnano.5.184 -
J. Phys. Chem. C 119(41), 23752-23766 (2015),
doi: 10.1021/acs.jpcc.5b06809 -
Cryst. Eng. Comm. 17(45), 8612-8622 (2015),
doi: 10.1039/C5CE01388G -
Cryst. Eng. Comm. 17(45), 8565 (2015),
doi: 10.1039/C5CE90198G -
Angew. Chem. Int. Ed. 54(47), 13912-13917 (2015),
doi: 10.1002/anie.201505512 -
Inorg. Chem. 54(22), 10701-10710 (2015),
doi: 10.1021/acs.inorgchem.5b01593

