| Authors: | Ahmed M. Rozza, Danny E. P. Vanpoucke, Eva-Maria Krammer, Julie Bouckaert, Ralf Blossey, Marc F. Lensink, Mary Jo Ondrechen, Imre Bakó, Julianna Oláh, and Goedele Roos |
| Journal: | Journal of Molecular Liquids 384, 122172 (2023) |
| doi: | 10.1016/j.molliq.2023.122172 |
| IF(2021): | 6.633 |
| export: | bibtex |
| pdf: | <JMolLiq> |
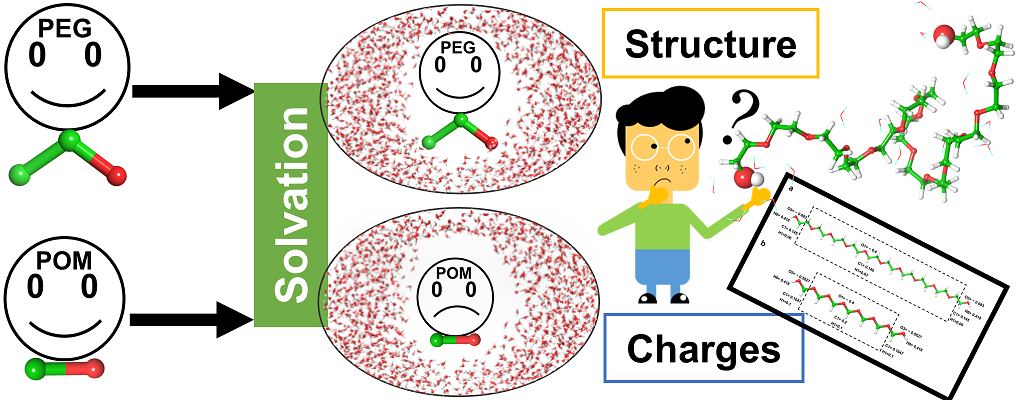 |
| Graphical Abstract: PEG or POM, similar in structure though very different in their solvation. Is this due to structure or charge(distribution)? |
Abstract
Non-toxic, chemically inert, organic polymers as polyethylene glycol (PEG) and polyoxymethylene (POM) have versatile applications in basic research, industry and pharmacy. In this work, we aim to characterize the hydration structure of PEG and POM oligomers by exploring how the solute disturbs the water structure compared to the bulk solvent and how the solute chain interacts with the solvent. We explore the effect of (i) the C-C-O (PEG) versus CO (POM) constitution of the chain and (ii) chain length. To this end, MD simulations followed by clustering and topological analysis of the hydration network, as well as by quantum
mechanical calculations of atomic charges are used. We show that the hydration varies with chain conformation and length. The degree of folding of the chain impacts its degree of solvation, which is measurable by different parameters as for example the number of water molecules in the first solvation shell and the solvent accessible surface. Atomic charges calculated on the oligomers in gas phase are stable throughout conformation and chain length and seem not to determine solvation. Hydration however induces charge transfer from the solute molecule to the solvent, which depends on the degree of hydration.