Tag: MIL-47
| Authors: |
Danny E. P. Vanpoucke |
| Journal: |
J. Phys. Chem. C 121(14), 8014-8022 (2017) |
| doi: |
10.1021/acs.jpcc.7b01491 |
| IF(2017): |
4.484 |
| export: |
bibtex |
| pdf: |
<J.Phys.Chem.C> |
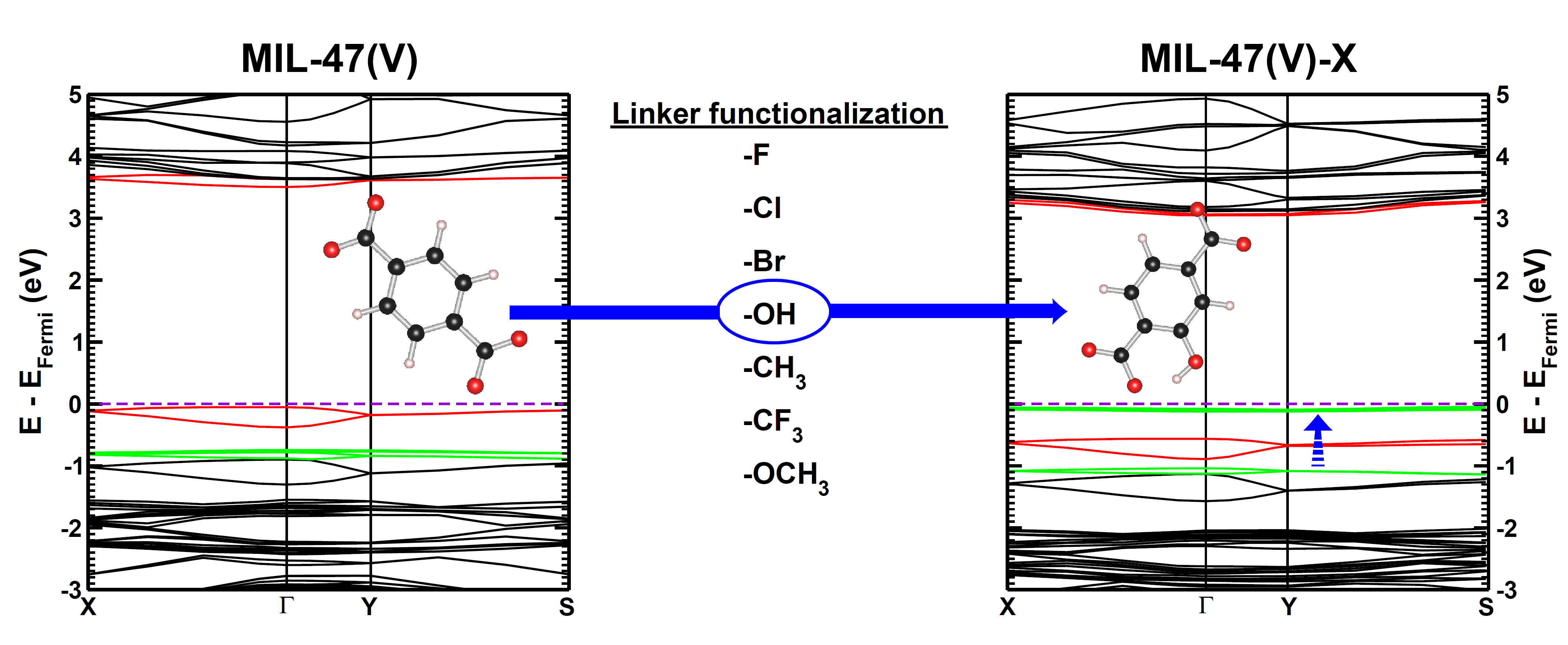 |
| Graphical Abstract: Evolution of the electronic band structure of MIL-47(V) upon OH-functionalization of the BDC linker. The π-orbital of the BDC linker splits upon functionalisation, and the split-off π-band moves up into the band gap, effectively reducing the latter. |
Metal–organic frameworks (MOFs) have gained much interest due to their intrinsic tunable nature. In this work, we study how linker functionalization modifies the electronic structure of the host MOF, more specifically, the MIL-47(V)-R (R = −F, −Cl, −Br, −OH, −CH3, −CF3, and −OCH3). It is shown that the presence of a functional group leads to a splitting of the π orbital on the linker. Moreover, the upward shift of the split-off π-band correlates well with the electron-withdrawing/donating nature of the functional groups. For halide functional groups the presence of lone-pair back-donation is corroborated by calculated Hirshfeld-I charges. In the case of the ferromagnetic configuration of the host MIL-47(V+IV) material a half-metal to insulator transition is noted for the −Br, −OCH3, and −OH functional groups, while for the antiferromagnetic configuration only the hydroxy group results in an effective reduction of the band gap.
Permanent link to this article: https://dannyvanpoucke.be/mof-mil47-linkerfunct-en/
-
Filed under Cover
-
November 13, 2015
| Authors: |
Thomas Bogaerts, Louis Vanduyfhuys, Danny E.P. Vanpoucke, Jelle Wieme, Michel Waroquier, Pascal Van Der Voort, and Veronique Van Speybroeck, |
| Journal: |
CrystEngComm. 17(45), 8565 (2015) |
| doi: |
10.1039/C5CE90198G |
| IF(2015): |
3.849 |
| export: |
bibtex |
| pdf: |
<CrystEngComm> |
The cover image depicts an X-ray beam hitting a sample of MIL-47(V) Metal-Organic Framework (reddish powder), resulting in an X-ray diffraction pattern. This leads to the atomic structure on the left, Where the spin-densities are indicated for the anti-ferromagnetic ground state. (The related paper can be found here.)

Permanent link to this article: https://dannyvanpoucke.be/paper2015_xrdthomasdannycover-en/
| Authors: |
Danny E. P. Vanpoucke, Kurt Lejaeghere, Veronique Van Speybroeck, Michel Waroquier, and An
Ghysels |
| Journal: |
J. Phys. Chem. C 119(41), 23752-23766 (2015) |
| doi: |
10.1021/acs.jpcc.5b06809 |
| IF(2015): |
4.509 |
| export: |
bibtex |
| pdf: |
<J.Phys.Chem.C> |
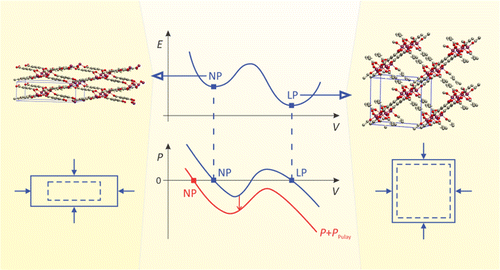 |
| Graphical Abstract: Pulay stresses complicate the structure optimization of the breathing MIL-47(V) Metal-Organic Framework. |
Modeling the flexibility of metal–organic frameworks (MOFs) requires the computation of mechanical properties from first principles, e.g., for screening of materials in a database, for gaining insight into structural transformations, and for force field development. However, this paper shows that computations with periodic density functional theory are challenged by the flexibility of these materials: guidelines from experience with standard solid-state calculations cannot be simply transferred to flexible porous frameworks. Our test case, the MIL-47(V) material, has a large-pore and a narrow-pore shape. The effect of Pulay stress (cf. Pulay forces) leads to drastic errors for a simple structure optimization of the flexible MIL-47(V) material. Pulay stress is an artificial stress that tends to lower the volume and is caused by the finite size of the plane wave basis set. We have investigated the importance of this Pulay stress, of symmetry breaking, and of k-point sampling on (a) the structure optimization and (b) mechanical properties such as elastic constants and bulk modulus, of both the large-pore and narrow-pore structure of MIL-47(V). We found that, in the structure optimization, Pulay effects should be avoided by using a fitting procedure, in which an equation of state E(V) (EOS) is fit to a series of energy versus volume points. Manual symmetry breaking could successfully lower the energy of MIL-47(V) by distorting the vanadium–oxide distances in the vanadyl chains and by rotating the benzene linkers. For the mechanical properties, the curvature of the EOS curve was compared with the Reuss bulk modulus, derived from the elastic tensor in the harmonic approximation. Errors induced by anharmonicity, the eggbox effect, and Pulay effects propagate into the Reuss modulus. The strong coupling of the unit cell axes when the unit cell deforms expresses itself in numerical instability of the Reuss modulus. For a flexible material, it is therefore advisible to resort to the EOS fit procedure.
Permanent link to this article: https://dannyvanpoucke.be/paper2015_accuratemofs-en/
| Authors: |
Thomas Bogaerts, Louis Vanduyfhuys, Danny E. P. Vanpoucke, Jelle Wieme,
Michel Waroquier, Pascal van der Voort and Veronique van Speybroeck |
| Journal: |
Cryst. Eng. Comm. 17(45), 8612-8622 (2015) |
| doi: |
10.1039/c5ce01388g |
| IF(2015): |
3.849 |
| export: |
bibtex |
| pdf: |
<Cryst.Eng.Comm.> |
 |
| Graphical Abstract: Which model represents the experimental XRD-spectra best? Ferromagnetic or anti-ferromagnetic chains? With of without offset? |
The structural characterization of complex crystalline materials such as metal organic frameworks can prove a very difficult challenge both for experimentalists as for theoreticians. From theory, the flat potential energy surface of these highly flexible structures often leads to different geometries that are energetically very close to each other. In this work a distinction between various computationally determined structures is made by comparing experimental and theoretically derived X-ray diffractograms which are produced from the materials geometry. The presented approach allows to choose the most appropriate geometry of a MIL-47(V) MOF and even distinguish between different electronic configurations that induce small structural changes. Moreover the techniques presented here are used to verify the applicability of a newly developed force field for this material. The discussed methodology is of significant importance for modelling studies where accurate geometries are crucial, such as mechanical properties and adsorption of guest molecules.
Permanent link to this article: https://dannyvanpoucke.be/paper2015_xrd_crystendcomm-en/
| Authors: |
Danny E. P. Vanpoucke, Jan W. Jaeken, Stijn De Baerdemacker, Kurt Lejaeghere
and Veronique Van Speybroeck |
| Journal: |
Beilstein J. Nanotechnol. 5, 1738-1748 (2014) |
| doi: |
10.3762/bjnano.5.184 |
| IF(2014): |
2.670 |
| export: |
bibtex |
| pdf: |
<Beilstein> (open access) |
 |
| Graphical Abstract: The MIL-47(V) MOF has one unpaired electron per V site. As a result, different spin configurations are possible, several of which lead to an anti-ferromagnetic state. The spin density of an antiferromagnetic state, containing only ferromagnetic chains is shown on the left. On the right, the electronic band structure of the same system is presented. |
The geometric and electronic structure of the MIL-47(V) metal-organic framework (MOF) is investigated by using ab initio density functional theory (DFT) calculations. Special focus is placed on the relation between the spin configuration and the properties of the MOF. The ground state is found to be antiferromagnetic, with an equilibrium volume of 1554.70 Å3. The transition pressure of the pressure-induced large-pore-to-narrow-pore phase transition is calculated to be 82 MPa and 124 MPa for systems with ferromagnetic and antiferromagnetic chains, respectively. For a mixed system, the transition pressure is found to be a weighted average of the ferromagnetic and antiferromagnetic transition pressures. Mapping DFT energies onto a simple-spin Hamiltonian shows both the intra- and inter-chain coupling to be antiferromagnetic, with the latter coupling constant being two orders of magnitude smaller than the former, suggesting the MIL-47(V) to present quasi-1D behavior. The electronic structure of the different spin configurations is investigated and it shows that the band gap position varies strongly with the spin configuration. The valence and conduction bands show a clear V d-character. In addition, these bands are flat in directions orthogonal to VO6 chains, while showing dispersion along the the direction of the VO6 chains, similar as for other quasi-1D materials.
Permanent link to this article: https://dannyvanpoucke.be/paper2014_mil47beil-en/
| Authors: |
Shyam Biswas, Danny E. P. Vanpoucke, Toon Verstraelen, Matthias Vandichel, Sarah Couck, Karen Leus, Ying-Ya Liu, Michel Waroquier, Veronique Van Speybroeck, Joeri F. M. Denayer, and Pascal Van Der Voort |
| Journal: |
J. Phys. Chem. C 117(44), 22784-22796 (2013) |
| doi: |
10.1021/jp406835n |
| IF(2013): |
4.835 |
| export: |
bibtex |
| pdf: |
<J.Phys.Chem.C> |
Six new functionalized vanadium hydroxo terephthalates [VIII(OH)(BDC-X)]·n(guests) (MIL-47(VIII)-X-AS) (BDC = 1,4-benzenedicarboxylate; X = −Cl, −Br, −CH3, −CF3, −OH, −OCH3; AS = as-synthesized) along with the parent MIL-47 were synthesized under rapid microwave-assisted hydrothermal conditions (170 °C, 30 min, 150 W). The unreacted H2BDC-X and/or occluded solvent molecules can be removed by thermal activation under vacuum, leading to the empty-pore forms of the title compounds (MIL-47(VIV)-X). Except pristine MIL-47 (+III oxidation state), the vanadium atoms in all the evacuated functionalized solids stayed in the +IV oxidation state. The phase purity of the compounds was ascertained by X-ray powder diffraction (XRPD), diffuse reflectance infrared Fourier transform (DRIFT) spectroscopy, Raman, thermogravimetric (TG), and elemental analysis. The structural similarity of the filled and empty-pore forms of the functionalized compounds with the respective forms of parent MIL-47 was verified by cell parameter determination from XRPD data. TGA and temperature-dependent XRPD (TDXRPD) experiments in an air atmosphere indicate high thermal stability in the 330–385 °C range. All the thermally activated compounds exhibit significant microporosity (SBET in the 305–897 m2 g–1 range), as verified by the N2 and CO2 sorption analysis. Among the six functionalized compounds, MIL-47(VIV)-OCH3 shows the highest CO2 uptake, demonstrating the determining role of functional groups on the CO2 sorption behavior. For this compound and pristine MIL-47(VIV), Widom particle insertion simulations were performed based on ab initio calculated crystal structures. The theoretical Henry coefficients show a good agreement with the experimental values, and calculated isosurfaces for the local excess chemical potential indicate the enhanced CO2 affinity is due to two effects: (i) the interaction between the methoxy group and CO2 and (ii) the collapse of the MIL-47(VIV)-OCH3 framework.
Permanent link to this article: https://dannyvanpoucke.be/paper2013_mofbiswas-en/






