Convergence of Atomic Charges with the Size of the Enzymatic Environment
| Authors: |
Danny E. P. Vanpoucke, Julianna Oláh, Frank De Proft, Veronique Van Speybroeck, and Goedele Roos |
| Journal: |
J. Chem. Inf. Model. 55(3), 564-571 (2015) |
| doi: |
10.1021/ci5006417 |
| IF(2015): |
3.657 |
| export: |
bibtex |
| pdf: |
<J.Chem.Inf.Model.> |
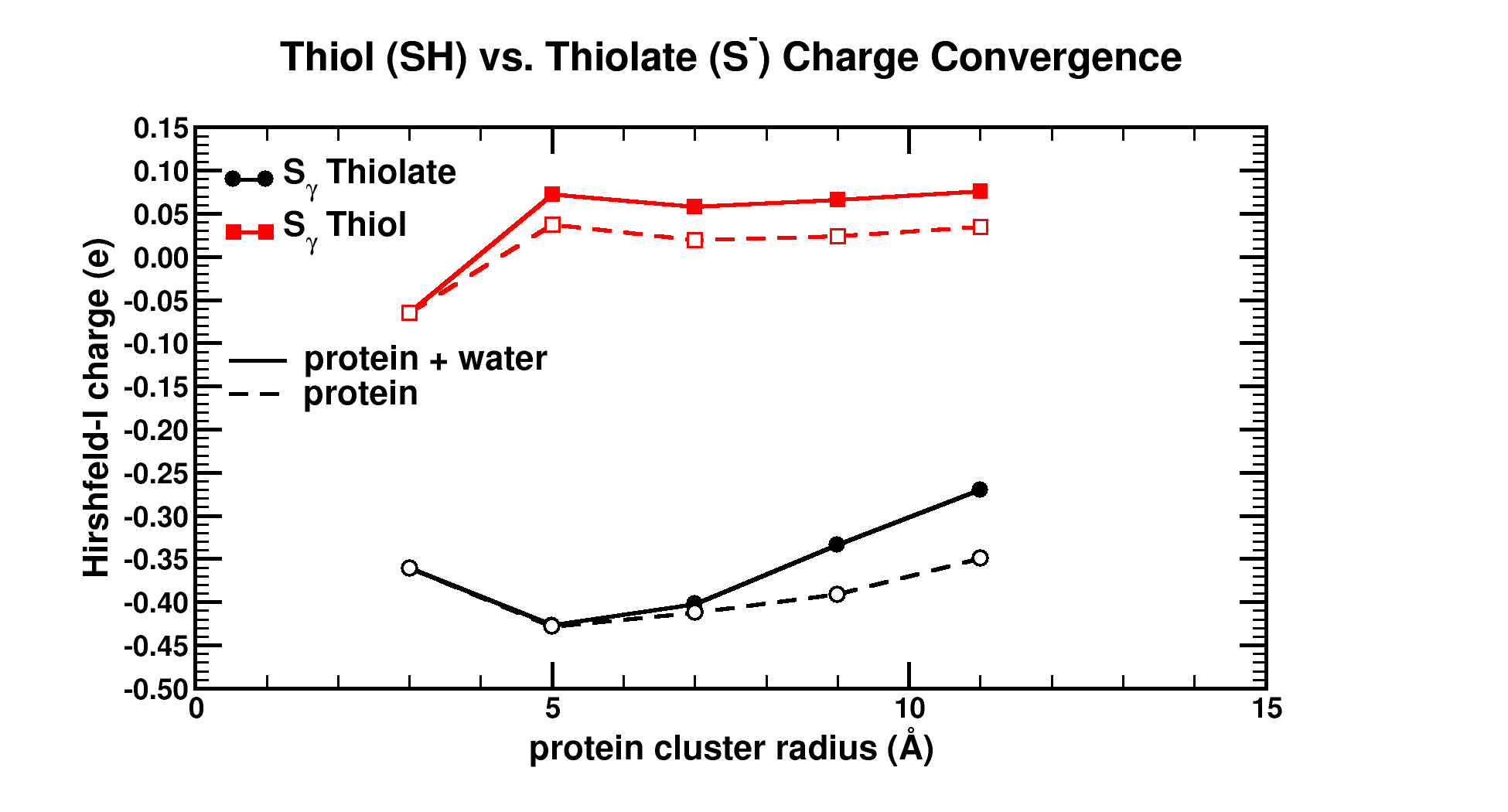 |
| Graphical Abstract: Graphical Abstract: The influence of the cluster size and water presence on the atomic charge of active and inactive sites in Bio-molecules. |
Atomic charges are a key concept to give more insight into the electronic structure and chemical reactivity. The Hirshfeld-I partitioning scheme applied to the model protein human 2-cysteine peroxiredoxin thioredoxin peroxidase B is used to investigate how large a protein fragment needs to be in order to achieve convergence of the atomic charge of both, neutral and negatively charged residues. Convergence in atomic charges is rapidly reached for neutral residues, but not for negatively charged ones. This study pinpoints difficulties on the road towards accurate modeling of negatively charged residues of large bio-molecular systems in a multiscale approach.
Permanent link to this article: https://dannyvanpoucke.be/paper2015_jcheminfo-en/


