Most commented posts
- Phonons: shake those atoms — 3 comments
- Start to Fortran — 1 comment
Dec 20 2016
One of the main differences between theory and computational research is the fact that the latter has to deal with finite resources; mainly time and storage. Where theoretical calculations involve integrations over continuous spaces, infinite sums and basis sets, computational work performs numerical integrations as weighted sums over finite grids and cuts of infinite series. As an infinite number of operations would take an infinite amount of time, it is clear why numerical evaluations are truncated. If the contributions of an infinite series become smaller and smaller, it is also clear that at some point the contributions will become smaller than the numerical accuracy, so continuation beyond that point is …pointless.
In case of ab initio quantum mechanical calculations, we aim to get as accurate results at an as low computational cost as possible. Even with the current availability of computational resources, an infinite sum would still take an infinite amount of time. In addition, although parallelization can help out a lot in getting access to additional computational resources during the same amount of real time, codes are not infinitely parallel, so at some point adding more CPU’s will no longer speed up the calculations. Two important parameters in quantum mechanical calculations to play with are the basis set size (Or kinetic energy cut off, in case of plane wave basis sets. In which case this can also be related to the real space integration grid) and the integration grid for the reciprocal space (the k-point grid).
These two parameters are not unique to VASP, they are present in all quantum mechanical codes, but we will use VASP as an example here. The example system we will use is the α-phase of Cerium, using the PBE functional. The default cut-off energy used by VASP is 299 eV.
What a basis set is and how it is defined depends strongly on the code. As such you are referred to the manual/tutorials of your code of interest.(VASP workshop) One important thing to remember, however, is the fact that although a plane wave basis set is “nicely behaved” (bigger basis = more accurate result) this is not true for all types of basis sets (Gaussian basis sets are an important example here).
How do you perform a convergence test?
This does not need to be a fully optimized geometry, an experimental geometry or a reasonable manually constructed geometry will do fine, as long as it gives you a converged result at the end of your static calculation. A convergence test should not depend on the exact geometry of your system. Rather it should tell you how well your setting converges your result with regard to the energy found on the potential energy surface.
(to reasonable values, although the settings should—to make your life somewhat sane—be independent with regard to convergence testing).
VASP specific parameters of importance:
These should be a simple static calculation. Make sure that each of the calculations finishes successfully, otherwise you will not be able to compare results and check convergence.
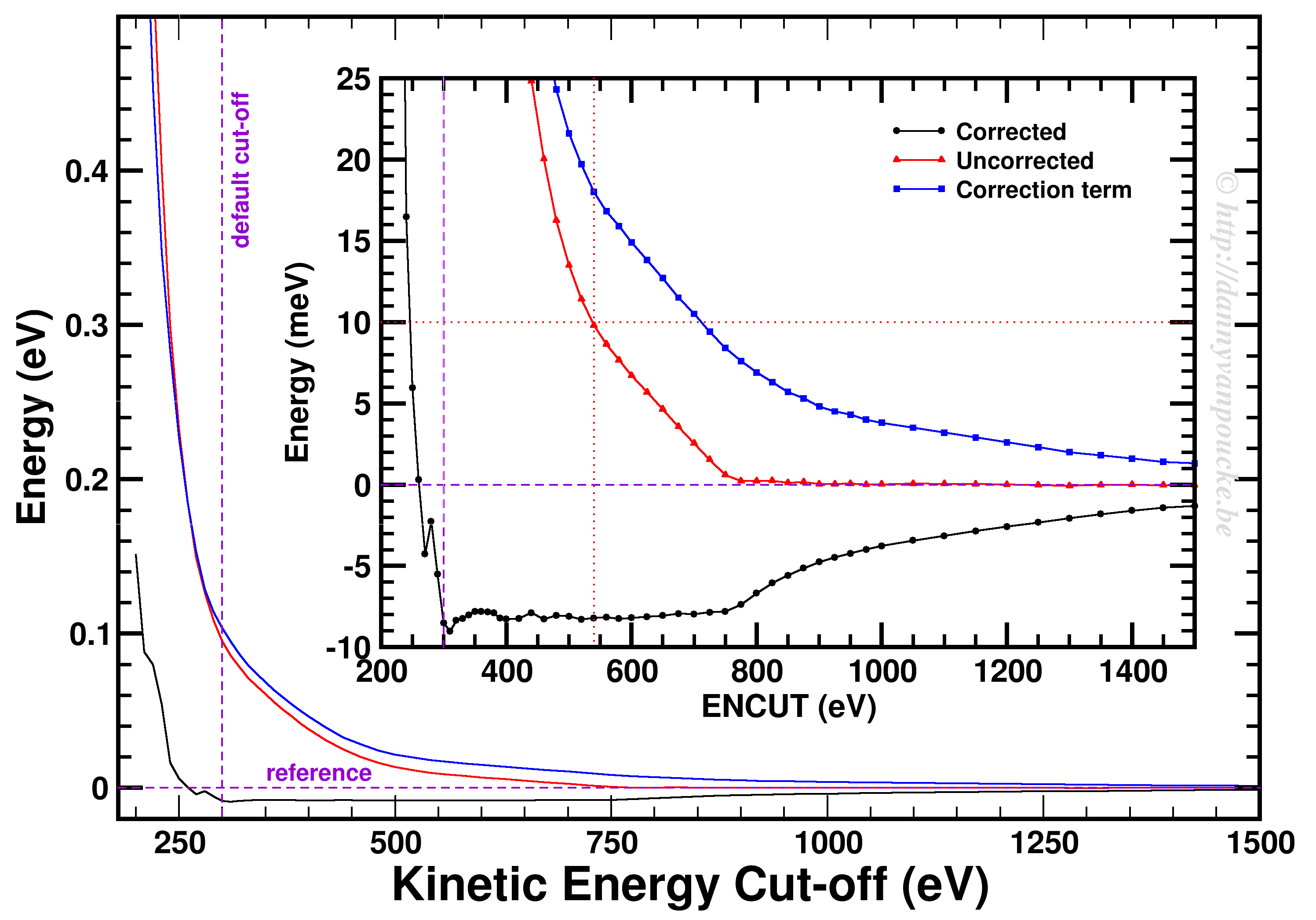
Convergence of the kinetic energy cut-off for alpha Ce using the PBE functional and a 9x9x9 k-point grid.
In our example, we used a 9x9x9 k-point set. Looking at the example, we first of all see how smoothly the total energy varies with regard to the ENCUT parameter. In addition, it is important to note that VASP has a correction term (search for EATOM in the OUTCAR file) implemented which greatly improves the energy convergence (compare the black and red curves). Unfortunately, it also leads to non-variational convergence (i.e. the energy does not become strictly smaller with increasing cut-off) which may lead to some confusion. However, the correction term performs really well, and allows you to use a kinetic energy cut-off which is much lower than what you would need to use without. In this case, the default cut-off misses the reference energy by about 10 meV. Without the correction, a cut-off of about 540 eV (almost double) is needed. From ENCUT=300 to 800 eV, you observe a plateau, so using a higher cut-off will not improve the energy much. However, other properties, such as the calculated forces or the hessian may improve in this region. For these parameters a higher cut-off may be beneficial, and their convergence as function of ENCUT should be checked if important for your work.
Similar as for the kinetic energy cut-off, if you are working with a periodic system you should check the convergence of your k-point set. However, if you are working with molecules/clusters your Brillouin zone reduces to a single point, so your k-point set should only consist of the Gamma point and no convergence testing is needed. More importantly, if you use a larger k-point set for such systems (molecules/clusters) you introduce artificial interaction between the periodic copies which should be avoided at all cost.
For bulk materials a k-point convergence check has a similar setup as the basis set convergence check. The main difference being the fact that for these calculation the basis set is kept constant (VASP: ENCUT = default cut-off, manually set) and the k-point set is varied. As such, if you are new to quantum mechanical calculations, or start using a new code, you can combine the two convergence checks and study the convergence behavior on a 2D surface.
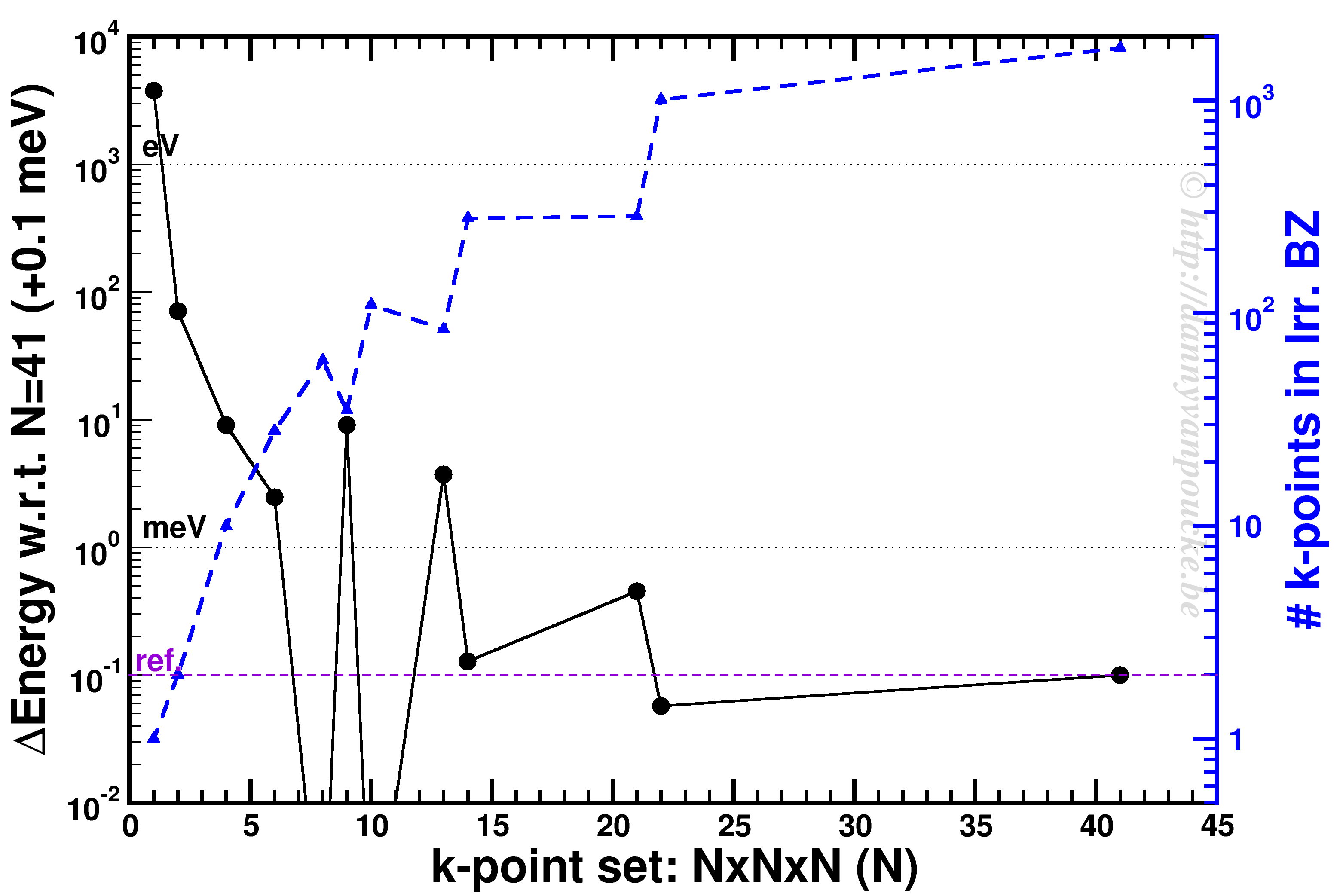
K-point convergence of alpha-Cerium using the PBE functional and ENCUT=500 eV.
In our example, ENCUT was set to 500 eV. It is clear that an extended k-point set is important for small systems, as the Gamma-point only energy can be off by several eV. This is even the case for some large systems like MOFs. An important thing to remember with regard to k-point convergence, is the fact that this convergence is not strictly declining, it may show significant oscillations overshooting and undershooting the converged value. A convergence of 1 meV or less for the entire system is a goal to aim for. An exception may be the most large systems, but even then one should keep in mind the size of the energy barriers in that system. Using flexible MOFs as an example which show a large-pore to narrow-pore transition barrier of 10-20 meV per formula unit, k-point convergence should be much below this. Otherwise your system may accidentally cross this barrier during relaxation.
The blue curve shows the number of k-points in the irreducible Brillouin zone. For standard density functional theory calculations (LDA and GGA, not hybrid functionals) this is a measure of the computational cost, as the k-points can be calculated fully independently in parallel (and yes the blue scale is a log-scale as well). Because the first orders of magnitude in accuracy are quickly crossed ( from Gamma to 6x6x6 the energy error goes from the order of eV to meV) while the number of k-points doesn’t grow that quickly (from 1 to 28). As a result, one often performs structure optimizations in a stepped fashion, starting with a coarse grid steadily increasing the grid (unless pathological behavior is expected… MOFs again…yes, they do leave you with nightmares in this regard).
Convergence testing is necessary, in theory, for each and every new system you look into. Luckily, VASP behaves rather nicely, such that over time you will know what to expect and your convergence tests will reduce in size significantly and become more focused. In the examples above we used the total energy as a reference, but this is not always the most important aspect to consider. In some cases you should check the convergence as function of the accuracy of the forces. In that case you generally will end up with more stringent criteria as energy converges rather nicely and quickly.
May your convergence curves be smooth and quick.
Dec 13 2016

Bart Sorée receives a commemorative frame of the event. Foto courtesy of Rajesh Ramaneti.
Today I have the pleasure of chairing the last symposium of the year of the MRS chapter at UHasselt. During this invited lecture, Bart Sorée (Professor at UAntwerp and KULeuven, and alumnus of my own Alma Mater) will introduce us into the topic of topological insulators.
This topic became unexpectedly a hot topic as it is part of the 2016 Nobel Prize in Physics, awarded last Saturday.
This year’s Nobel prize in physics went to: David J. Thouless (1/2), F. Duncan M. Haldane (1/4) and J. Michael Kosterlitz (1/4) who received it
On the Nobel Prize website you can find this document which gives some background on this work and explains what it is. Beware that the explanation is rather technical and at an abstract level. They start with introducing the concept of an order parameter. You may have heard of this in the context of dynamical systems (as I did) or in the context of phase transitions. In the latter context, order parameters are generally zero in one phase, and non-zero in the other. In overly simplified terms, one could say an order parameter is a kind of hidden variable (not to be mistaken for a hidden variable in QM) which becomes visible upon symmetry breaking. An example to explain this concept.
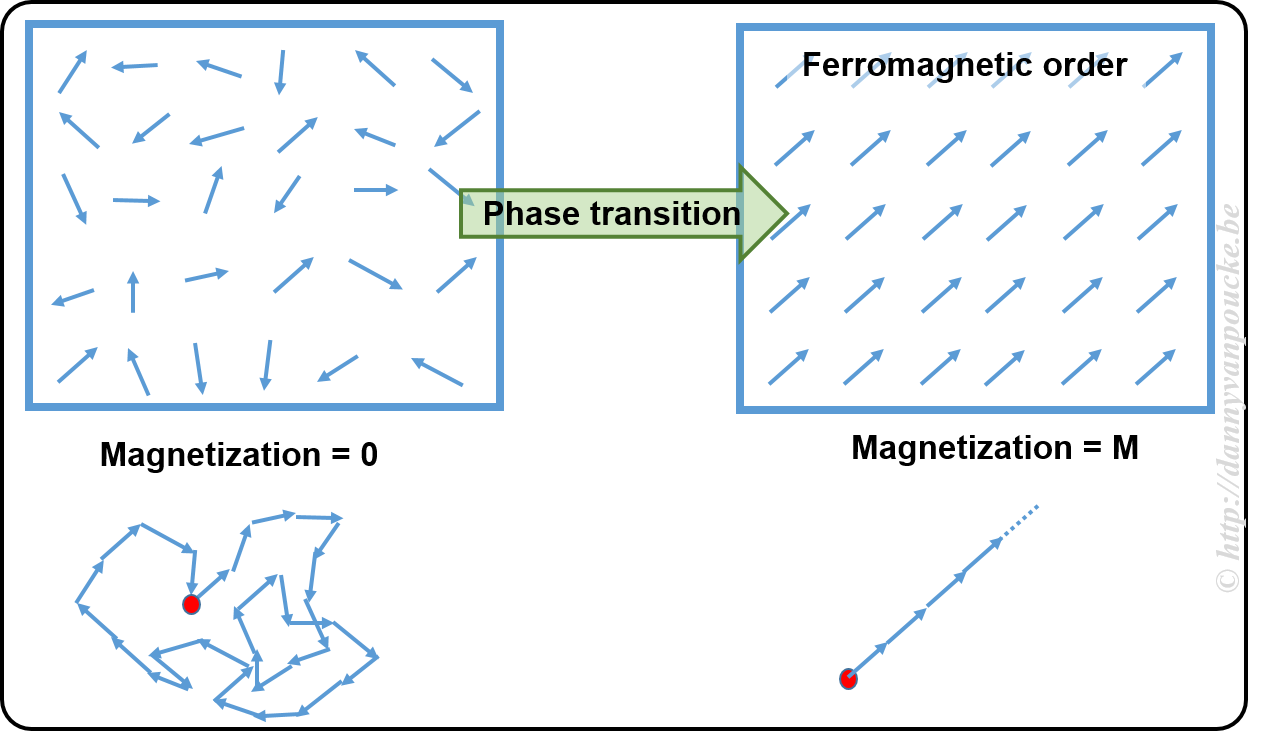
In a ferromagnetic material, the atoms have what is called a spin (imagine it as a small magnetic needle pointing in a specific direction, or a small arrow). At high temperature these spins point randomly in all possible directions, leading to a net zero magnetization (the sum of all the small arrows just lets you run in circles going nowhere). This magnetization is the order parameter. At the high temperature, as there is no preferred direction, the system is invariant under rotation and translations (i.e. if you shift it a bit or you rotate it, or both you will not see a difference) When the temperature is lower, you will cross what is called a critical temperature. Below this temperature all spins will start to align themselves parallel, giving rise to a non-zero magnetization (if all arrows point in the same direction, their sum is a long arrow in that direction). At this point, the system has lost the rotational invariance (because all spins point in direction, you will know when someone rotated the system) and the symmetry is said to have broken.
Within the context of phase transitions, order parameters are often temperature dependent. In case of topological materials this is not the case. A topological material has a topological order, which means both phases are present at absolute zero (or the temperature you will never reach in any experiment no matter how hard you try) or maybe better without the presence of temperature (this is more the realm of computational materials science, calculations at 0 Kelvin actually mean without temperature as a parameter). So the order parameter in a topological material will not be temperature dependent.
To complicate things, topological insulators are materials which have a topological order which is not as the one defined above 😯 —yup why would we make it easy 🙄 . It gets even worse, a topological insulator is conducting.
OK, before you run away or loose what is remaining of your sanity. A topological insulator is an insulating material which has surface states which are conducting. In this it is not that different from many other “normal” insulators. What makes it different, is that these surface states are, what is called, symmetry protected. What does this mean?
In a topological insulator with 2 conducting surface states, one will be linked to spin up and one will be linked to spin down (remember the ferromagnetism story of before, now the small arrows belong to the separate electrons and exist only in 2 types: pointing up=spin up, and pointing down=spin down). Each of these surface states will be populated with electrons. One state with electrons having spin up, the other with electrons having spin down. Next, you need to know that these states also have a real-space path let the electrons run around the edge of material. Imagine them as one-way streets for the electrons. Due to symmetry the two states are mirror images of one-another. As such, if electrons in the up-spin state more left, then the ones in the down-spin state move right. We are almost there, no worries there is a clue. Now, where in a normal insulator with surface states the electrons can scatter (bounce and make a U-turn) this is not possible in a topological insulator. But there are roads in two directions you say? Yes, but these are restricted. And up-spin electron cannot be in the down-spin lane and vice versa. As a result, a current going in such a surface state will show extremely little scattering, as it would need to change the spin of the electron as well as it’s spatial motion. This is why it is called symmetry protected.
If there are more states, things get more complicated. But for everyone’s sanity, we will leave it at this. 😎
Nov 23 2016
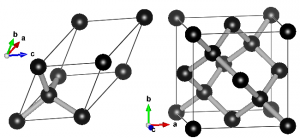
Ball-and-stick representation of diamond in two different unit cells. Left: primitive unit cell containing two atoms. All atoms at the vertices are periodic copies of the same one. Right: Conventional cubic unit cell containing eight atoms. Atoms at opposing faces are periodic copies, while all atoms at the vertices are periodic copies of the same atom.
When performing electronic structure calculations on complex systems, you prefer to do this on systems with as few atoms as possible. Such periodic cells are called unit cells. There are, however, two types of unit cells: Primitive unit cells and conventional unit cells.
A primitive unit cell is the smallest possible periodic cell of a crystalline material, making it extremely suited for calculations. Unfortunately, it is not always the nicest unit cell to work with, as it may be difficult to recognize it’s symmetry (cf. the example of diamond on the right). The conventional unit cell on the other hand shows the symmetry more clearly, but is not (always) the smallest possible unit cell. To make matters complicated and confusing, people often refer to both types as simply “unit cell”, which is not wrong, but the term unit cell is for many uniquely associated with only one of the two types.
When you are performing calculations on diamond, the conventional cell isn’t that large that standard calculations become impossible, even on a personal laptop or desktop. On the other hand, when you are studying a Metal-Organic Framework like the UiO-66(Zr) which contains 456 atoms in its conventional unit cell, you will be very happy to use the primitive unit cell with ‘merely’ 114 atoms. Also the MIL-47/53 topology which generally is studied using a conventional unit cell containing 72/76 can be reduced to a smaller primitive unit cell of only 36/38 atoms. Just as for the diamond primitive unit cell, this MIL47/53 primitive unit cell is not a nice cubic cell. Instead you end up with a lattice having lattice angles of seventy-something degrees.
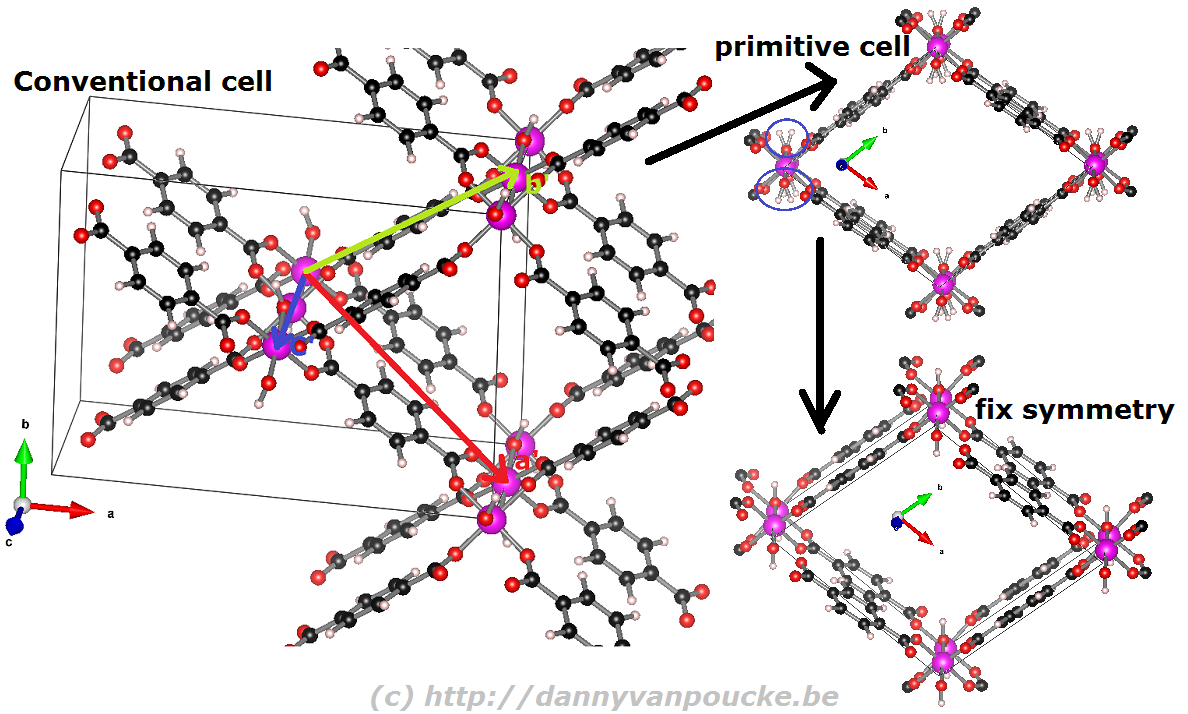
Reduction of the MIL-53 conventional cell to the primitive cell. The conventional cell is shown, extending slightly into the periodic copies. The primitive lattice vectors are shown as colored arrows. The folded primitive cell shows there was some symmetry-breaking in the hydroxy groups of the metal-oxide chain. Introducing some additional symmetry fixes this in the final primitive cell.
Before you start, and if you are using VASP, make sure you have the POSCAR file giving the atomic positions as Cartesian coordinates. (Using the HIVE-4 toolbox: Option TF, suboption 2 (Dir->Cart).)
If you do not use VASP, you can still make use of the scheme below.
 |
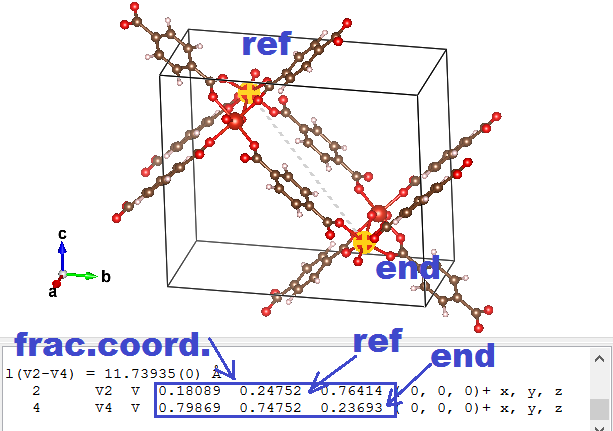 |
⇒ aprim = axprim,frac aconv + ayprim,frac bconv + azprim,frac cconv
So imagine that the lattice vectors of the MOF above are a = ( 20, 0, 0), b = ( 0, 15, 0), and c = ( 0, 0, 5). And the primitive fractional a vector is found to be aprim,frac = ( 0.5, -0.5, 0.5). In this case the aprim vector will become: aprim = ( 10, 0, 0 ) + ( 0, -7.5, 0 ) + ( 0, 0, 2.5) = (10, -7.5, 2.5).
As you can see, the method is quite simple and straight forward, albeit a bit tedious if you need to do this many times.
PS: Small remark for those new to VESTA. You can use delete atoms in VESTA and store your structure again. This is useful if you want to play with a molecule. Unfortunately for a solid you need also to get new lattice vectors, which did not happen. As a result you end up with some atoms floating around in a periodically repeated box with the original lattice parameters. Steps 1-5 given above provide a simple way of not ending up in this situation, but require some typing on your part.
PS 2: The opposite transformation, from a primitive unit cell to a conventional unit cell, using VESTA, is shown in this youtube video.
Nov 16 2016
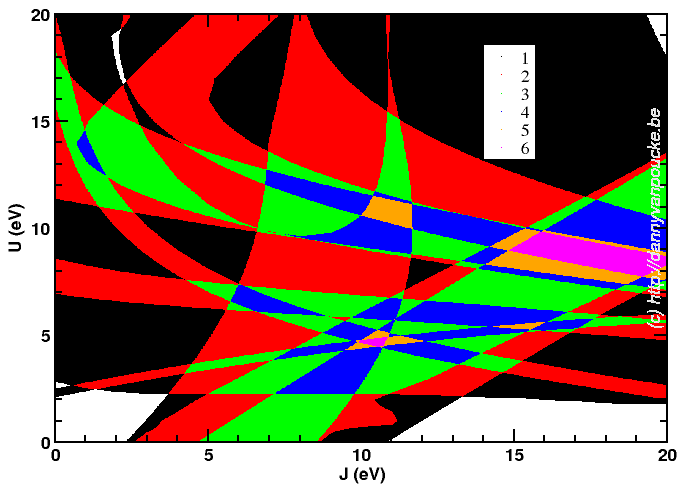
Although it looks a bit like a modern piece of art, it is one more attempt at trying to find an optimum combination of parameters.
I’m currently trying to find “the best choice” for U and J for a DFT+U based project… DFT??? Density Functional Theory. This is an approximate method which is used in computational materials science to calculate the quantum mechanical behavior of electrons in matter. Instead of solving the Schrödinger equation, known from any quantum mechanic course, one solves the Hohenberg-Kohn-Sham equations. In these equations it are not the electrons which play a central role (which they do in the Schrödinger equations) but the electron density. Hohenberg, Kohn and Sham were able to show that their equations give the exact same results as the Schrödinger equations. There is, however, one small caveat: you need to have an “exact” exchange-correlation functional (a functional is just a function of a function). Unfortunately there is no known analytic form for this functional, so one needs to use approximated functionals. As you probably guessed, with these approximate functionals the solution of the Hohenberg-Kohn-Sham equations is no longer an exact solution.
For some molecules or solids the error is much larger than average due to the error in the exchange-correlation functional. These systems are therefore called “strongly-correlated” systems. Over the years, several ways have been devised to solve this problem in DFT. One of them is called DFT+U. It entails adding additional coulomb interactions (Hubbard-U-potential) between the “strongly interacting electrons”. However this additional interaction depends on the system at hand, so one always needs to fit this parameter against one of more properties one is interested in. The law of conservation of misery, however, makes sure that improving one property goes hand in hand with a deterioration of another property.
Since actual DFT+U has two independent parameters (U and J, though for many systems they can be dependent reducing to a single parameter) I had quite some fun running calculations for a 21×21 grid of possible pairs. Afterward, collecting the data I wanted to use for fitting purposes took my script about 2h! 😯 Unfortunately the 10 properties of interest I wanted to fit give optimum (U,J)-pair all over the grid. In the picture above, you see my most recent attempt at trying to deal with them. It shows for the entire grid how many of the 10 properties are reasonably well fit.There are two regions which fit 6 properties; One around (U,J)=(5,10) and another around (U,J)=(8.5,17.5). There will be more work before this gives a satisfactory result, the show will go on.
Oct 18 2016
 Yesterday was a good day for computational scientists in Flanders. The new TIER-1 machine, named BrENIAC, located at the university of Leuven, was inaugurated and is now officially open to all users of the Flemish university associations: UAntwerpen, VUB, UGhent, UHasselt, and KULeuven. The name refers to one of the first (super)computers ever built: ENIAC. This new machine will take over the task of the first TIER-1 machine (muk, located at the university of Ghent), which will be decommissioned at the end of this year. BrENIAC is ranked 196th in the current top 500 of supercomputers, and costs 5.5 M€. This is of course without the annual cost of power usage and technical personnel which will maintain the machine and provide support for the scientists running calculations. With its 580 compute nodes, containing 28 cores each (or 2 14-core CPU’s of the type Broadwell E5-2680v4), the number of available cores has roughly doubled. Also memory access should have improved, which gives rise to a theoretical threefold increase of the peak performance.
Yesterday was a good day for computational scientists in Flanders. The new TIER-1 machine, named BrENIAC, located at the university of Leuven, was inaugurated and is now officially open to all users of the Flemish university associations: UAntwerpen, VUB, UGhent, UHasselt, and KULeuven. The name refers to one of the first (super)computers ever built: ENIAC. This new machine will take over the task of the first TIER-1 machine (muk, located at the university of Ghent), which will be decommissioned at the end of this year. BrENIAC is ranked 196th in the current top 500 of supercomputers, and costs 5.5 M€. This is of course without the annual cost of power usage and technical personnel which will maintain the machine and provide support for the scientists running calculations. With its 580 compute nodes, containing 28 cores each (or 2 14-core CPU’s of the type Broadwell E5-2680v4), the number of available cores has roughly doubled. Also memory access should have improved, which gives rise to a theoretical threefold increase of the peak performance.
However, this peak performance is measured with “benchmark” tests, which tend to behave much better than real life programs. This is because the average scientific programmer doesn’t write the best optimized code (ok, “commercial” programs these days may even behave worse :p ) for various reasons, time constraints being one of them. So my first task, before I start running my simulations on the new TIER-1 machine, will be to benchmark VASP and my own HIVE-code.
Two videos of my new sidekick:
You can see me in my front-row position in this picture taken during the non-academic part of the inauguration.
Oct 06 2016
Yesterday was the tUL Life Sciences Research Day 2016. A conference event build around finding collaboration possibilities between the University of Hasselt in Belgium and the University of Maastricht (The Netherlands)…after all tUL is the “transnational University Limburg” which brings two universities together that are only separated some 26 km, but you have to cross a national border.
Although Life sciences itself is not my personal niche, I went to look for opportunities, as nano-particles which are used for drug delivery often consist of metals or oxides. These materials on the other hand are my niche. I used my current work on MOFs as a means to show what is possible from the ab-initio point of view, and presented this as a poster.

Poster presented at the tUL Life Sciences Research Day, depicting my work on the unfunctionalized and the functionalized MIL-47(V) MOF.
Sep 25 2016
| Authors: | S. A. Rounaghi, H. Eshghi, S. Scudino, A. Vyalikh, D. E. P. Vanpoucke, W. Gruner, S. Oswald, A. R. Kiani Rashid, M. Samadi Khoshkhoo, U. Scheler and J. Eckert |
| Journal: | Scientific Reports 6, 33375 (2016) |
| doi: | 10.1038/srep33375 |
| IF(2016): | 4.259 |
| export: | bibtex |
| pdf: | <Sci.Rep.> (open access) |
Hexagonal Aluminium nitride (h-AlN) is an important wide-bandgap semiconductor material which is conventionally fabricated by high temperature carbothermal reduction of alumina under toxic ammonia atmosphere. Here we report a simple, low cost and potentially scalable mechanochemical procedure for the green synthesis of nanostructured h-AlN from a powder mixture of Aluminium and melamine precursors. A combination of experimental and theoretical techniques has been employed to provide comprehensive mechanistic insights on the reactivity of melamine, solid state metalorganic interactions and the structural transformation of Al to h-AlN under non-equilibrium ball milling conditions. The results reveal that melamine is adsorbed through the amine groups on the Aluminium surface due to the long-range van der Waals forces. The high energy provided by milling leads to the deammoniation of melamine at the initial stages followed by the polymerization and formation of a carbon nitride network, by the decomposition of the amine groups and, finally, by the subsequent diffusion of nitrogen into the Aluminium structure to form h-AlN
Aug 31 2016

Visit to Stockholm. The knight at the Medeltidsmuseet (top left), brown bear in Skansen (top right), visiting the Royal palace (bottom left) and local entertainment in the old city center (bottom right).
Summertime is a time of rest for most people. For our little academic family, last summer was a bit of a roller coaster; alternating holidays with hard work which had been postponed too much. The last vestige of my start of a new chapter (moving the remaining stuff from the apartment to our house) was finally bested. Now the conference roller coaster has started with Sylvia’s plenary lecture on conceptual spaces in Stockholm.
As neither of us ever visited Sweden before, we decided to turn it into a semi-family-holiday as well. Our 4-year-old son enjoyed his first ever plane flight (he wasn’t really convinced something impressive was going on). And while Sylvia was of to the conference, the two of us went to explore Stockholm: Finding the knight in the Medeltidsmuseet (at the left in the back of this beautiful museum 🙂 ) and searching for the king and queen at their palace (they weren’t there 🙁 ). Or visiting one of the oldest open-air musea; Skansen (similar to Bokrijk in Belgium) where we saw old professions at work (making cheese for example) and native Scandinavian farm and wild animals (from peacocks to brown bears).
Next weekend starts the next episode of the conference roller-coaster with me hosting a 2-day colloquium on porous frameworks together with Bartek Szyja and Ionut Tranca at the CMD-26 conference in Groningen. We have a nicely packed colloquium with about 20 presentations (8 invited and 12 contributed) covering the whole realm of porous materials from zeolites to COFs and MOFs. The program of the colloquium can be downloaded below: